Beijing Kanghong Biomedical Co.,Ltd.
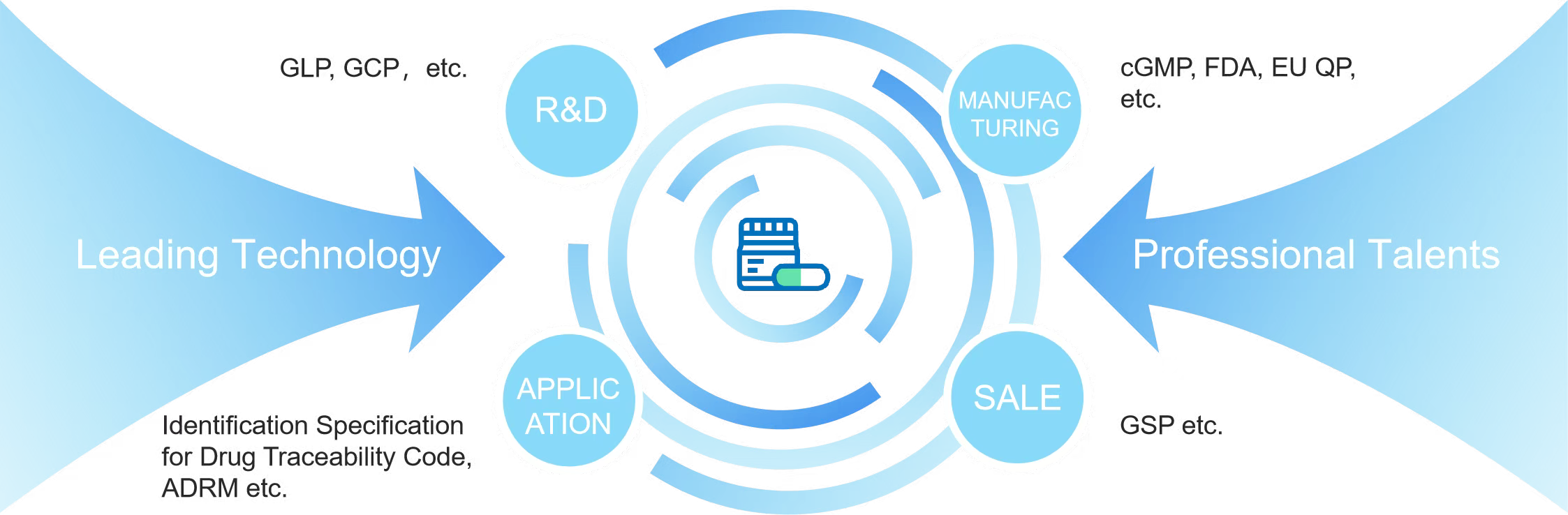
Beijing Kanghong Biomedical Co.,Ltd. includes a biopharmaceutical commercial manufacturing plant and supporting facilities designed and constructed according to NMPA GMP and EMA&FDA cGMP regulations,and the total area is 140,000m².
This manufature site facilitates the technological advances and new product production.